Fourth dose COVID vaccine
Until now the FDA had allowed a fourth vaccine dose only for the immune-compromised as young as 12. Of the 1050 eligible health care workers enrolled in the Sheba HCW COVID-19 Cohort 1 2 154 received the fourth dose of BNT162b2 and 1 week later 120 received mRNA-1273.

Eu Members Question Fourth Dose Except Most Vulnerable Euractiv Com
Download the Moderna COVID-19 Vaccine fact sheet for more information.
. The 4th dose or 2nd booster COVID-19 vaccines train the bodys immune system to elicit a rapid response to SARS-CoV-2. They induce an immune response involving the. Stay informed during the severe weather season with.
Americans 50 and older can get a second COVID-19 booster if its been at least four months since their last vaccination. Food and Drug Administration FDA issued an emergency use authorization EUA for a fourth dose of Pfizer-BioNTechs and Modernas mRNA vaccines. Fourth dose is a booster shot Also in August the Food and Drug Administration FDA authorized the third additional vaccine dose for people who are immunocompromised.
The Centers for Disease Control and Prevention quickly. Countries are beginning to offer a fourth dose of the Covid-19 vaccine to vulnerable groups but medical professionals are undecided on whether it would benefit the. The team found that 14 days after the fourth jab antibody levels against SARS-CoV-2 the virus that causes COVID-19 jumped 16-fold in those who were boosted with.
This comes as Israel is offering a fourth dose of COVID-19 vaccination to those who work in healthcare and people who are over age 60. No new safety concerns. Compared with a third vaccine dose a fourth dose of the PfizerBioNTech COVID-19 vaccine lowered the risk of infection symptomatic infection hospitalization severe illness.
For the Moderna vaccine the fourth booster shot will be half the dose from the primary series. Currently a fourth dose is available to anyone aged over 65 Aboriginal and Torres Strait Islanders over 50 and people who are severely immunocompromised. If you choose to receive your fourth dose second booster as early as three months 84 days after your first booster please book this dose through the Provincial Vaccine.
Tue 24 May 2022 717am. The 4th dose or 2nd booster COVID-19 vaccines train the bodys immune system to elicit a rapid response to SARS-CoV-2. Our results indicate that a fourth dose of BNT162b2 vaccine increases protection against PCR-confirmed SARS-CoV-2 infection symptomatic Covid-19 Covid-19related.
3rd dose given at least 4 weeks 28 days after 2nd dose Booster and Timing. Ad Learn about Emergency Use Authorization EUA and why it was granted. Download the Moderna COVID-19 Vaccine fact sheet for more information.
Only the Pfizer vaccine can be used in those as young as 12. For the Moderna vaccine. Find a COVID-19 Vaccine.
Search vaccinesgov text your ZIP code to 438829 or call 1-800-232-0233 to find locations near you. In this open-label nonrandomized clinical study we assessed the immunogenicity and safety of a fourth dose of either BNT162b2 PfizerBioNTech or mRNA-1273 Moderna. Immunocompromised People Should Get a Fourth Shot The CDC recommends that moderately or severely immunocompromised individuals who received a two-dose mRNA.
William Schaffner an expert in. A fourth dose of a COVID-19 mRNA vaccine can boost antibodies and other immune responses to levels higher than those seen after the third dose according to UK trial. Not recommended at this time Pre-teens Teens and Adults Who Are Moderately or Severely.
For example this tweet from RapTV claims that the CEO of Pfizer said that a fourth dose of their COVID-19 vaccine would be necessary Thats it. Check your local pharmacys website to see if. On March 29 the US.
A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19-related outcomes among persons who had received a third dose at least 4 months earlier. Who can get a fourth COVID jab. The Australian Technical Advisory Group on Immunisation ATAGI has recommended an additional vaccine dose for the following groups.
Ad Learn about Emergency Use Authorization EUA and why it was granted. The Food and Drug Administration has authorized a fourth Pfizer and Moderna dose for people age 50 and older. The tweet offered no.
The 05 mL 100 micrograms dosage is used for all three doses of the primary. The fourth dose increased neutralizing antibody levels against the coronavirus compared to the levels people had 5 months after the first booster.
Who Is Eligible For A Fourth Covid 19 Vaccine Dose Across Canada The Globe And Mail
Fourth Shot Protects Against Severe Omicron Outcomes Covid May Increase Risk Of Rare Eye Blood Clots Reuters
Chile A Vaccine Front Runner Launches Fourth Covid Dose Reuters

Covid Vaccines It S Too Early To Consider Giving People A Fourth Dose Say Eu Officials Euronews
Omicron Pfizer Ceo Says We May Need Fourth Covid Vaccine Doses Sooner Than Expected
Cdc Recommends Fourth Pfizer And Moderna Covid Vaccine Doses For People Age 50 And Older
Israel Broadens Eligibility For Fourth Shot Of Covid Vaccine Reuters
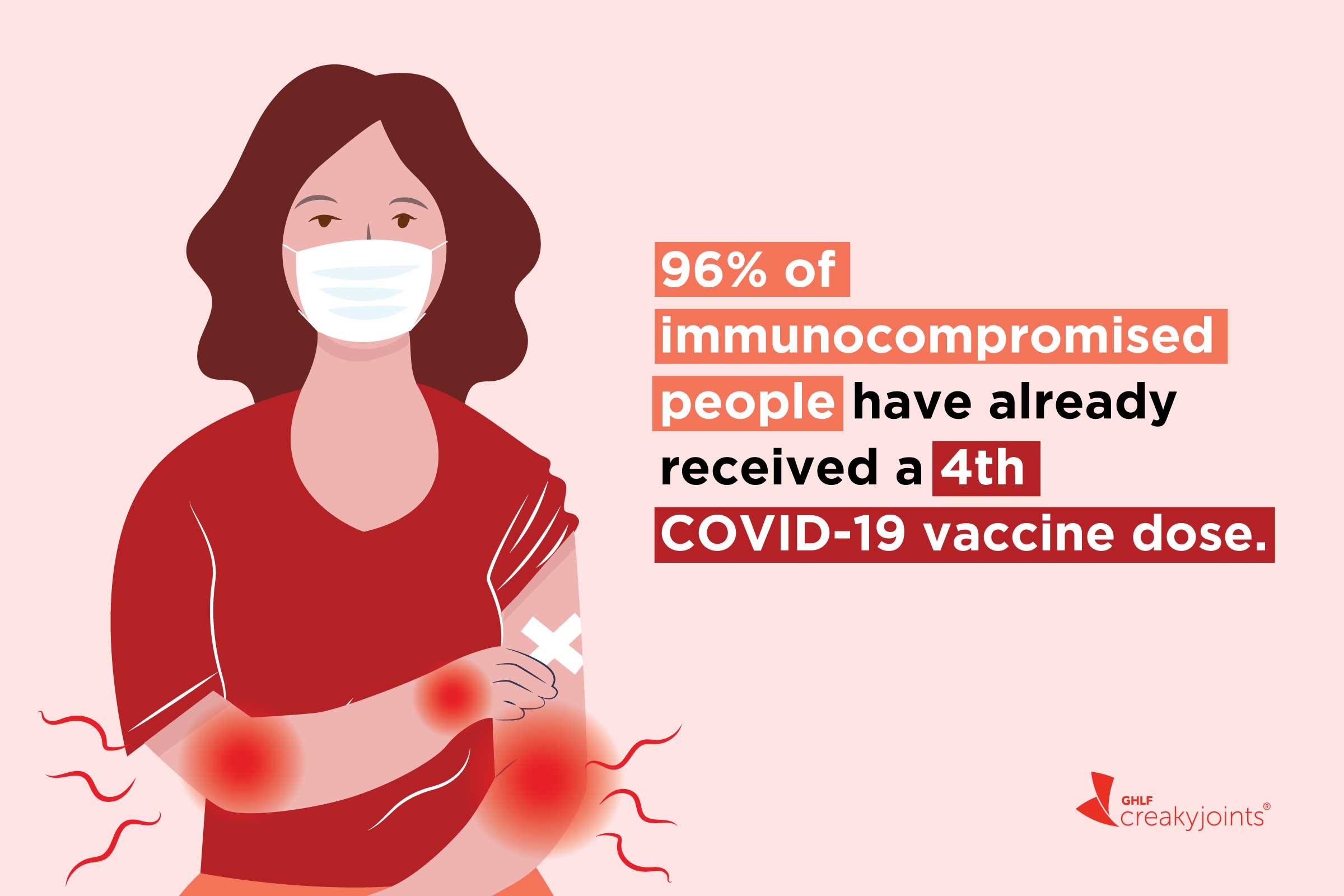
Most Immunocompromised People Have Gotten The 4th Covid 19 Vaccine Dose

Cdc Some Immunocompromised People Can Get A Fourth Dose Medpage Today
Opinion Before Canada Focuses On Fourth Covid 19 Shots Let S Get Third Doses To Everyone The Globe And Mail

Italy Recommends Fourth Dose For Immunocompromised Patients Euractiv Com

Experts Worry Messaging May Hinder Fourth Dose Uptake Citynews Halifax
/cloudfront-us-east-1.images.arcpublishing.com/gray/NY6G5YPNTRBL7FKBLYD7OXLTGA.jpg)
Fact Finders Who Is Eligible For The Fourth Dose Of The Vaccine

Pfizer Asks Us To Allow 4th Covid Vaccine Dose For Seniors

Fourth Dose Of Covid Vaccine Offers Only Slight Boost Against Omicron Infection

With Covid Cases Surging Across Australia Will A Fourth Vaccine Dose Be Required Coronavirus The Guardian

Will States Offer 4th Covid Vaccine Doses West Virginia First To Ask Cdc For Permission